Strep throat is a common and treatable childhood disease in the U.S., but in less wealthy countries, children afflicted with strep can develop rheumatic fever, in which runaway inflammation attacks the body’s tissues. Rheumatic fever often damages the valves of the heart, causing rheumatic heart disease that can lead to serious health problems, including heart failure. Heart valves can be surgically replaced, but children whose bodies are still growing may need multiple, highly invasive surgeries to replace their valves with larger ones, putting them at risk.
Kevin Kit Parker’s team at the Wyss Institute and Harvard’s John A. Paulson School of Engineering and Applied Sciences (SEAS) committed to fix this problem by creating an implantable heart valve that grows with a child, minimizing surgical complications and suffering.
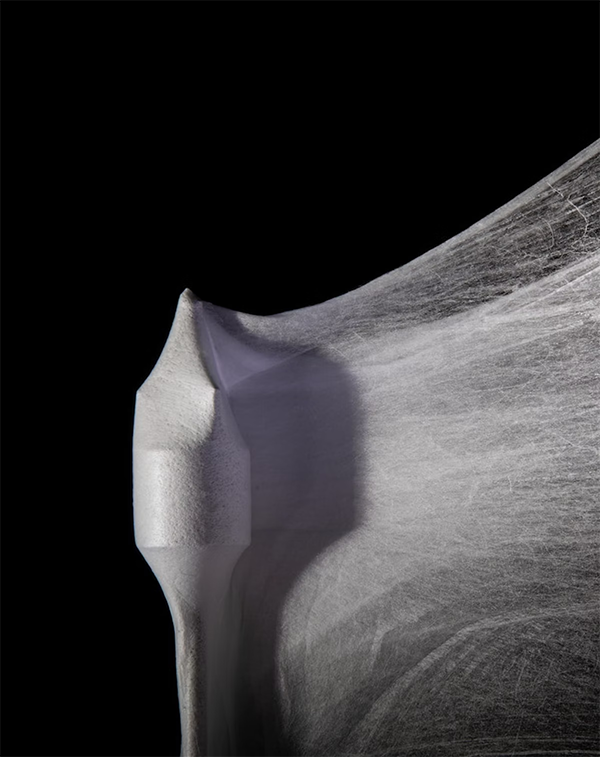
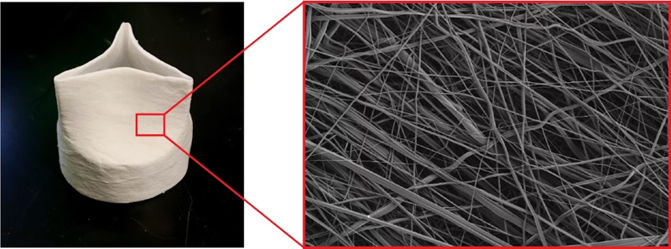
The team is a big step closer to that goal, as described in a new paper published in Matter. Their next-generation synthetic heart valve, called FibraValve, can be manufactured in less than 10 minutes using a new method called focused rotary jet spinning (FRJS), which allows the researchers to customize the shape and properties of the valve’s delicate flaps down to the nanoscale.
This new valve was readily colonized by living cells, both in vitro and in large animal model studies conducted by collaborators led by Wyss Associate Faculty member Simon Hoerstrup at the Wyss Zurich Translational Center in Zurich, Switzerland. This international collaboration was supported by Wyss Institute Project funding.
“This study illustrates FibraValves’ potential as a solution for children suffering from valve diseases. Our goal is for the patient’s native cells to use the device as a blueprint to regenerate their own living valve tissue, but FRJS also has potential as a platform to fabricate other medical devices in the future,” said Parker.
Parker and Hoerstrup’s quest to create a living, growing heart valve has been underway since 2014, and they produced their first synthetic heart valve, JetValve, in 2017. For the new FibraValve manufacturing process, they added streams of focused air to the polymer-extruding stream to create focused rotary jet spinning (FRJS). This allowed them to improve the rate at which the polymer fibers were deposited onto the mandrel, and to easily adjust the resulting valve to the final desired shape. The polymer also produced networks of micro- and nano-fibers that better mimicked the tissue structure of a human heart valve.
The team also used a new, custom polymer material to improve the infiltration of living cells once the FibraValve is implanted into the body. This material, called PLCL, is a combination of polycaprolactone (PCL) and polylactic acid (PLA). Previous studies had shown that PLCL lasts about six months when implanted into rats, is infiltrated by living cells and remodeled into functional tissue, and safely biodegrades.
 TEXTILES.ORG
TEXTILES.ORG


